Generic Medicines
Taj Pharma is the largest generic pharmaceutical company in India. We hold top positions in different established markets worldwide generics markets..
(Methadone Hydrochloride Tablets, USP)
also available:
Dispersible Tablets
Methadone Hydrochloride Tablets USP, 40 mg
DESCRIPTION
Methadone Hydrochloride Tablets for Oral Suspension USP is for oral administration following dispersion in a liquid. Each tablet contains 40 mg of methadone hydrochloride.
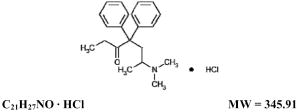
Methadone hydrochloride is chemically described as (3-heptanone, 6-(dimethylamino)-4,4- diphenyl-, hydrochloride). Methadone hydrochloride is a white, essentially odorless, bitter-tasting crystalline powder. It is very soluble in water, soluble in isopropanol and in chloroform, and practically insoluble in ether and in glycerine. It is present in methadone hydrochloride tablets for oral suspension as the racemic mixture. Methadone hydrochloride has a melting point of 235°C, a pKa of 8.25 in water at 20°C, a solution (1 in 100) pH between 4.5 and 6.5, a partition coefficient of 117 at pH 7.4 in octanol/water and a molecular weight of 345.91. Its molecular formula is C21H27NO•HCl and its structural formula is:
The preparation of methadone hydrochloride for oral suspension contains insoluble excipients and must not be injected.
Each Methadone Hydrochloride Tablet for Oral Suspension USP contains:
Methadone Hydrochloride USP...............40 mg (0.116 mmol)
In addition, each METHADOSE® Dispersible Tablet also contains: dibasic calcium phosphate USP; microcrystalline cellulose NF; magnesium stearate NF; colloidal silicon dioxide NF; pregelatinized starch NF; stearic acid NF.
Each Methadone Hydrochloride Tablet USP (Dispersible, Orange Flavored) also contains: colloidal silicon dioxide NF; dibasic calcium phosphate dihydrate USP; magnesium stearate NF; microcrystalline cellulose NF; pregelatinized starch NF; stearic acid NF; orange blend: FD&C yellow #6, FD&C yellow #6 lake, and FD&C yellow #5 lake; orange flavor.
CLINICAL PHARMACOLOGY
Mechanism of Action
Methadone hydrochloride is a mu-agonist; a synthetic opioid analgesic with multiple actions qualitatively similar to those of morphine, the most prominent of which involves the central nervous system and organs composed of smooth muscle. The principal therapeutic uses for methadone are analgesia and detoxification or maintenance in opioid addiction. The methadone abstinence syndrome, although qualitatively similar to that of morphine, differs in that the onset is slower, the course is more prolonged, and the symptoms are less severe.
Some data also indicate that methadone acts as an antagonist at the N-methyl-D-aspartate (NMDA) receptor. The contribution of NMDA receptor antagonism to methadone's efficacy is unknown. Other NMDA receptor antagonists have been shown to produce neurotoxic effects in animals.
Pharmacokinetics
Pharmacokinetics in Special Populations
INDICATIONS AND USAGE
For detoxification treatment of opioid addiction (heroin or other morphine-like drugs).
For maintenance treatment of opioid addiction (heroin or other morphine-like drugs), in conjunction with appropriate social and medical services.
Note – Outpatient maintenance and outpatient detoxification treatment may be provided only by Opioid Treatment Programs (OTPs) certified by the Federal Substance Abuse and Mental Health Services Administration (SAMHSA) and registered by the Drug Enforcement Administration (DEA). This does not preclude the maintenance treatment of a patient with concurrent opioid addiction who is hospitalized for conditions other than opioid addiction and who requires temporary maintenance during the critical period of his/her stay, or of a patient whose enrollment has been verified in a program which has been certified for maintenance treatment with methadone.
CONTRAINDICATIONS
Methadone hydrochloride for oral suspension is contraindicated in patients with a known hypersensitivity to methadone hydrochloride or any other ingredient in methadone hydrochloride for oral suspension.
Methadone is contraindicated in any situation where opioids are contraindicated such as: patients with respiratory depression (in the absence of resuscitative equipment or in unmonitored settings), and in patients with acute bronchial asthma or hypercarbia.
Methadone is contraindicated in any patient who has or is suspected of having a paralytic ileus.
WARNINGS
Respiratory Depression
Respiratory depression is the chief hazard associated with methadone hydrochloride administration. Methadone's peak respiratory depressant effects typically occur later, and persist longer than its peak analgesic effects, in the short-term use setting. These characteristics can contribute to cases of iatrogenic overdose, particularly during treatment initiation and dose titration.
Respiratory depression is of particular concern in elderly or debilitated patients as well as in those suffering from conditions accompanied by hypoxia or hypercapnia when even moderate therapeutic doses may dangerously decrease pulmonary ventilation.
Methadone should be administered with extreme caution to patients with conditions accompanied by hypoxia, hypercapnia, or decreased respiratory reserve such as: asthma, chronic obstructive pulmonary disease or cor pulmonale, severe obesity, sleep apnea syndrome, myxedema, kyphoscoliosis, and central nervous system (CNS) depression or coma. In these patients, even usual therapeutic doses of methadone may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea. Methadone should be used at the lowest effective dose and only under careful medical supervision.
Cardiac Conduction Effects
This information is intended to alert the prescriber to comprehensively evaluate the risks and benefits of methadone treatment. The intent is not to deter the appropriate use of methadone in patients with a history of cardiac disease.
Laboratory studies, both in vivo and in vitro, have demonstrated that methadone inhibits cardiac potassium channels and prolongs the QT interval. Cases of QT interval prolongation and serious arrhythmia (torsades de pointes) have been observed during treatment with methadone. These cases appear to be more commonly associated with, but not limited to, higher dose treatment (>200 mg/day). Although most cases involve patients being treated for pain with large, multiple daily doses of methadone, cases have been reported in patients receiving doses commonly used for maintenance treatment of opioid addiction. In most of the cases seen at typical maintenance doses, concomitant medications and/or clinical conditions such as hypokalemia were noted as contributing factors. However, the evidence strongly suggests that methadone possesses the potential for adverse cardiac conduction effects in some patients.
Methadone should be administered with particular caution to patients already at risk for development of prolonged QT interval (e.g., cardiac hypertrophy, concomitant diuretic use, hypokalemia, hypomagnesemia). Careful monitoring is recommended when using methadone in patients with a history of cardiac conduction abnormalities, those taking medications affecting cardiac conduction, and in other cases where history or physical exam suggest an increased risk of dysrhythmia. QT prolongation has also been reported in patients with no prior cardiac history who have received high doses of methadone. Patients developing QT prolongation while on methadone treatment should be evaluated for the presence of modifiable risk factors, such as concomitant medications with cardiac effects, drugs which might cause electrolyte abnormalities and drugs which might act as inhibitors of methadone metabolism.
The potential risks of methadone, including the risk of life-threatening arrhythmias, should be weighed against the risks of discontinuing methadone treatment. In the patient being treated for opiate dependence with methadone maintenance therapy, these risks include a very high likelihood of relapse to illicit drug use following methadone discontinuation.
The use of methadone in patients already known to have a prolonged QT interval has not been systematically studied. The potential risks of methadone should be weighed against the substantial morbidity and mortality associated with untreated opioid addiction.
In using methadone an individualized benefit to risk assessment should be carried out and should include evaluation of patient presentation and complete medical history. For patients judged to be at risk, careful monitoring of cardiovascular status, including evaluation of QT prolongation and dysrhythmias should be performed. Incomplete Cross-Tolerance between Methadone and Other Opioids
Patients tolerant to other opioids may be incompletely tolerant to methadone. Incomplete cross-tolerance is of particular concern for patients tolerant to other mu-opioid agonists who are being converted to methadone, thus making determination of dosing during opioid conversion complex. Deaths have been reported during conversion from chronic, high-dose treatment with other opioid agonists. A high degree of “opioid tolerance” does not eliminate the possibility of methadone overdose, iatrogenic or otherwise.
Misuse, Abuse, and Diversion of Opioids
Methadone is a mu-agonist opioid with an abuse liability similar to that of morphine and other opioid agonists and is a Schedule II controlled substance. Methadone, like morphine and other opioids used for analgesia, has the potential for being abused and is subject to criminal diversion.
Methadone can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing methadone hydrochloride for oral suspension in situations where the clinician is concerned about an increased risk of misuse, abuse, or diversion. Abuse of methadone poses a risk of overdose and death. This risk is increased with concurrent abuse of methadone with alcohol and other substances. In addition, parenteral drug abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.
Healthcare professionals should contact their State Professional Licensing Board or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Interactions with Other CNS Depressants
Patients receiving other opioid analgesics, general anesthetics, phenothiazines, other tranquilizers, sedatives, hypnotics, or other CNS depressants (including alcohol) concomitantly with methadone may experience respiratory depression, hypotension, profound sedation, or coma (see PRECAUTIONS).
Interactions with Alcohol and Drugs of Abuse
Methadone may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression. Deaths associated with illicit use of methadone frequently have involved concomitant benzodiazepine abuse.
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of opioids and their capacity to elevate cerebrospinal-fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, opioids produce effects which may obscure the clinical course of patients with head injuries. In such patients, methadone must be used with caution, and only if it is deemed essential.
Acute Abdominal Conditions
The administration of opioids may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
Hypotensive Effect
The administration of methadone may result in severe hypotension in patients whose ability to maintain normal blood pressure is compromised (e.g., severe volume depletion).
PRECAUTIONS
Methadone hydrochloride for oral suspension should be used with caution in elderly and debilitated patients; patients who are known to be sensitive to central nervous system depressants, such as those with cardiovascular, pulmonary, renal, or hepatic disease; and in patients with comorbid conditions or concomitant medications which may predispose to dysrhythmia or reduced ventilatory drive.
Drug Interactions
In vitro results suggest that methadone undergoes hepatic N-demethylation by cytochrome P450 enzymes, principally CYP3A4, CYP2B6, CYP2C19, and to a lesser extent by CYP2C9 and CYP2D6. Coadministration of methadone with inducers of these enzymes may result in a more rapid metabolism and potential for decreased effects of methadone, whereas administration with CYP inhibitors may reduce metabolism and potentiate methadone's effects. Although antiretroviral drugs such as efavirenz, nelfinavir, nevirapine, ritonavir, lopinavir+ritonavir combination are known to inhibit CYPs, they are shown to reduce the plasma levels of methadone, possibly due to their CYP induction activity. Therefore, drugs administered concomitantly with methadone should be evaluated for interaction potential; clinicians are advised to evaluate individual response to drug therapy.
Opioid Antagonists, Mixed Agonist/Antagonists, and Partial Agonists
As with other mu-agonists, patients maintained on methadone may experience withdrawal symptoms when given opioid antagonists, mixed agonist/antagonists, and partial agonists. Examples of such agents are naloxone, naltrexone, pentazocine, nalbuphine, butorphanol, and buprenorphine.
Information for Patients
Patients should be cautioned that methadone, like all opioids, may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving or operating machinery.
Patients should be cautioned that methadone, like other opioids, may produce orthostatic hypotension in ambulatory patients.
Patients should be cautioned that alcohol and other CNS depressants may produce an additive CNS depression when taken with this product and should be avoided.
Patients should be instructed to seek medical attention immediately if they experience symptoms suggestive of an arrhythmia (such as palpitations, dizziness, lightheadedness, or syncope) when taking methadone.
Patients initiating treatment with methadone should be reassured that the dose of methadone will “hold” for longer periods of time as treatment progresses.
Patients should be instructed to keep methadone in a secure place out of the reach of children and other household members. Accidental or deliberate ingestion by a child may cause respiratory depression that can result in death.
Patients should be advised not to change the dose of methadone without consulting their physician.
Women of childbearing potential who become or are planning to become pregnant should be advised to consult their physician regarding the effects of methadone use during pregnancy on themselves and their unborn child.
If a physically dependent patient abruptly discontinues use of methadone, an opioid abstinence or withdrawal syndrome may develop. If cessation of therapy is indicated, it may be appropriate to taper the methadone dose, rather than abruptly discontinue it, due to the risk of precipitating withdrawal symptoms. Their physician can provide a dose schedule to accomplish a gradual discontinuation of the medication.
Patients seeking to discontinue treatment with methadone for opioid dependence should be apprised of the high risk of relapse to illicit drug use associated with discontinuation of methadone maintenance treatment.
Patients should be advised that methadone is a potential drug of abuse. They should protect it from theft, and it should never be given to anyone other than the individual for whom it was prescribed.
Methadone hydrochloride for oral suspension is for oral administration only and must be initially dispersed in liquid before use. After dispersion in liquid, the preparation must not be injected.
